![]()
![]()
the threat of CFCs to the ozone layer in the stratosphere (1974) was explained
by Mario J. Molina Frank Sherwood Rowland in a paper for the journal Nature

the Miller-Urey experiment (1953)
the Turing mechanism (1952)
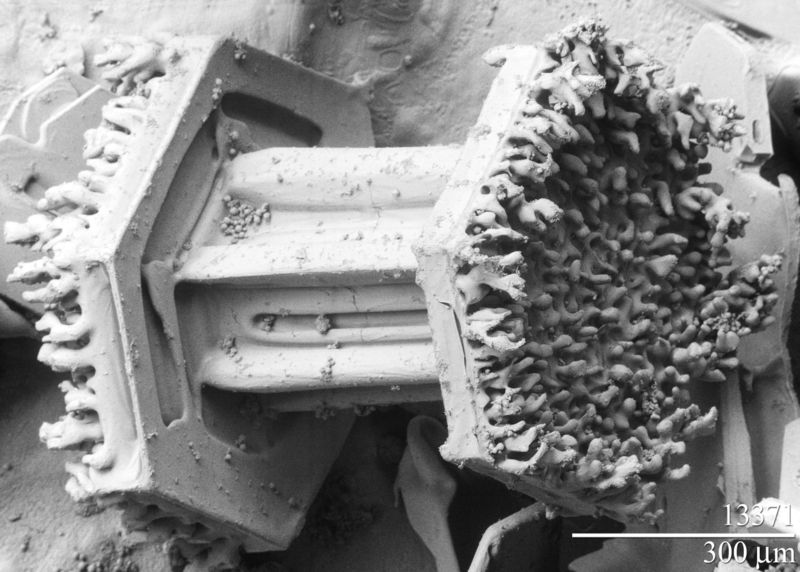
the electron microscope (1933)
Bohr's model of the atom was superseded by the probability cloud model of Erwin Schrödinger and Werner Heisenberg

Heisenberg's Uncertainty Principle (1927)
Lewis Acid-Base Theory (1923)
the existence of the neutron (1920) was suggested by William Draper Harkins.
Evidence for its existence was not obtained until 1932
Hermann Staudinger explained that polymeres (1920) are
long-chain molecules held together by ordinary valency bonds
the Czochralski process (1916)
Gilbert N. Lewis proposed the idea of covalent bonds (1916) and introduced the electron-dot notation
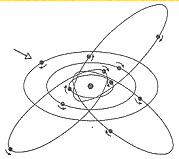
the Sommerfeld model of the atom (1916)
isotopes (1913) were discovered by J. J. Thomson
Moseley's law (1913)
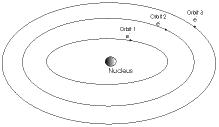
the Bohr model of the atom (1913)
the Rutherford model (1911)
the Geiger-Marsden experiment (1909)
the Henderson-Hasselbalch equation (1908)
the mole (1902)

the electron (1897) was discovered as a a subatomic particle by Joseph John Thomson. As a unit
of charge in electrochemistry the electron was posited by G. Johnstone Stoney already in 1874
the hydrogenation process (1897) was discovered by Paul Sabatier
the idea that changes in the levels of carbon dioxide in the atmosphere (1896) could substantially
alter the surface temperature through the greenhouse effect was first formulated by Svante Arrhenius
Svante Arrhenius' work on the conductivities of electrolytes (1884)
Karol Olszewski, Zygmunt Wróblewski and Karol Sitarski (1883) were the first to
liquefy oxygen, nitrogen and carbon dioxide from the atmosphere in a stable state
the Erlenmeyer Rule (1880)
Josiah Willard Gibbs' On the Equilibrium of Heterogeneous Substances (1876-1878) laid the foundations of physical chemistry
van der Waals' forces (1869)
the periodic table of elements (1869)
Dynamite (1866) was invented by Alfred Nobel
Avogadro's number (1865)
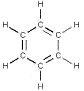
the carbon ring structure of benzene (1865) was proposed by Friedrich August Kekulé von Stradonitz
pasteurization (1862)
the Erlenmeyer flask (1861)
Stanislao Cannizzaro's Sunto di un corso di Filosofia chimica (1858)
the first aniline dye, mauveine (1856) was discovered accidentally by William Perkin
Johann Loschmidt (1856) determined the size of the molecules in air
Robert Bunsen started using a burner developed by his technician (1855) this burner quickly becomes
known as the Bunsen burner, although credit for its invention should really go to Michael Faraday
nitrocellulose (gun cotton) (1845) was acidentally discovered by Christian Schönbein
the first enzyme (1833) diastase was discovered by Anselme Payen
Urea (1828) was the first organic compound to be artificially synthesized from inorganic starting materials
hydrogen peroxide (1818) was discovered by Louis-Jacques Thénard
Jöns Jakob Berzelius developed the system of chemical notation (1813) in which
each element is represented by the initial letter or pairs of letters from their names
Amedeo Avogadro (1810) distinguished between atoms and molecules
Gay-Lussac's law (1809)

John Dalton's New System of Chemical Philosophy (1808)
Chemicals were classified as either organic or inorganic (1807) by the Swedish chemist Jöns Jakob Berzelius
the fact that acids and bases neutralise in equal proportions (1791) was discovered by Jeremias Richter
Joseph Priestley (1772) showed that growing plants can restore air
that has been made 'lifeless' by animals breathing it or fire burning in it
Joseph Black's experiments on magnesia, quicklime (1755) and other
alkaline substances was the first quantitative chemical research
carbon dioxide (1754) was discovered by Joseph Black
Daniel Gabriel Fahrenheit invented the first mercury thermometer (1714)
Boyle's law (1662)
the phlogiston theory (1660)
Andreas Libavius' Alchemia (1597)

Agricola's De re metallica (1556)
Paracelsus (1520) pioneered the use of chemicals and minerals in medicine
Hermeticism (1460)
Albertus Magnus isolated arsenic (1250)
Al-Razi's books on medicine and chemistry (925)

Jabir Ibn Hayyan (750) turned alchemy into a science

the Islamic Golden Age (700-1400)
the Emerald Tablets (300)